We are not just a machine manufacturer. We are your trusted partner for packaging integrity testing and analysis through feasibility studies, technical expertise, validation packages, product handling and custom-built solutions.
PDA Week
Date: 6 April - 11 April 2025
Location: Palm Springs Convention Center, Palm Springs, California
Booth Number: #118
Our Speaker: Gianmarco Pincelli, Technical Sales Manager
Session Title: Quality Inspection Methods for DIP Products According to Pharmacopoeia Annex I and the Benefits of Implementing AI Technology within Traditional Algorithms
Join Bonfiglioli Engineering at PDA Week 2025 at Booth 118 as we explore leading Automated Visual Inspection (AVI) and Leak Detection (CCIT) technologies for Difficult-to-Inspect Parenterals (DIP). Bonfiglioli Engineering is proud to be a featured manufacturer exhibiting their high-performance pharmaceutical packaging inspection systems within the STEQ America booth at PDA Week.
Join our technical expert, Gianmarco Pincelli, as he provides insights and expertise on AI-driven inspection solutions for DIP products. Discover how AI-driven inspection is transforming DIP quality assurance by improving defect detection, reducing false reject rates (FRR), and ensuring compliance with Annex I, USP 1207, and USP 1790.
Meet Our Expert Speaker
With extensive experience in automated visual inspection (AVI) and container closure integrity testing (CCIT), Gianmarco works closely with global pharmaceutical manufacturers to optimize quality assurance processes for their packaging including Difficult-to-Inspect Parenterals (DIP).
“The challenge of DIP inspection is its complexity. AI is revolutionizing how we approach visual inspection and leak detection—improving accuracy, reducing false rejects, and enhancing overall efficiency.”
Gianmarco Pincelli, Technical Sales Manager, Bonfiglioli Engineering
How This Session Can Help Your Pharmaceutical Container Operations
The increased complexity of modern pharmaceutical containers presents new challenges for automated visual inspection (AVI) and leak detection (CCIT). Regulations like Annex I require 100% inspection of parenterals for particulate contamination, making advanced inspection methods essential.
This educational session will address:
- The limitations of traditional silhouette-based AVI for DIP products
- How machine learning and neural networks improve AVI for BFS and powder-fill applications
- Reducing false reject rates (FRR) while ensuring regulatory compliance
- How AI-driven bubble and particulate detection enhances inspection accuracy
- Advanced leak detection methods (CCIT) for BFS and DIP packaging
If you’re looking to optimize DIP inspection and reduce waste, this session is essential.
"We’re excited to discuss how AI-driven AVI and CCIT can solve your most pressing inspection challenges. Stop by to learn more!" – Jesse Sklar, STEQ America
PDA Week Booth Details
We invite you to visit Bonfiglioli Engineering at the PDA Week exhibition hall, to meet Gianmarco Pincelli and Jesse Sklar from STEQ America for one-on-one discussions about:
- AI-enhanced Automated Visual Inspection (AVI) for DIP and BFS
- Leak Detection (CCIT) innovations for pharmaceutical packaging
- How to customize inspection systems to fit your production line’s needs
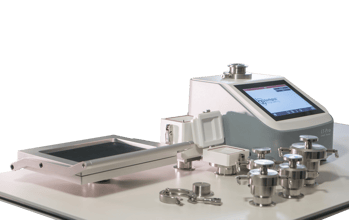
LT-Pro Leak Detector: Non-destructive benchtop CCIT solution for laboratory and in-process control applications.
Can’t Attend PDA Week? Let’s Connect!
Unable to attend PDA Week? We can bring the insights to you!
If you’re exploring ways to improve AVI and CCIT for DIP and BFS packaging, reach out to our team, today!
- Schedule a virtual consultation
- Email our team directly now
- Connect with Bonfiglioli Engineering on LinkedIn
- Follow on Bonfiglioli Engineering on YouTube




