Headspace Gas Analysis (HGA)
When monitoring container headspace conditions and their maintenance is critical to product quality and sterility in filled and finished packages. Read more below.
When monitoring container headspace conditions and their maintenance is critical to product quality and sterility in filled and finished packages. Read more below.
When monitoring container headspace conditions and their maintenance is critical to product quality and sterility in filled and finished packages. Read more below.
Many pharmaceuticals and food products are sealed into containers in a protective atmosphere to avoid contact with air that can damage quality and effectiveness. There are a diverse number of Container Closure Integrity Testing (CCIT) methods to guarantee proper sealing of containers like Vacuum Decay Method (VDM), Pressure Decay Method (PDM), Force Decay (FD), Lid Deflection.
Headspace analysis is classified under the CCIT procedure and is a laser-based, non-destructive and fully automatic inspection method for sealed packages that measures the quantity of certain gasses in the so-called “headspace” i.e. in the space inside the packaging not occupied by the product of an hermetically sealed package. It allows for measuring oxygen concentration, carbon dioxide, residual moisture content and absolute pressure value with the aim of verifying the headspace conditions and their maintenance to confirm stability and sterility in filled and finished packages.
HGA can be applied to a wide variety of containers like glass (tubular, molded, clear or amber), plastic and sterile products in the pharmaceutical, food and cosmetic industries.
Monitoring the maintenance of container headspace conditions is needed for sterile drugs such as oxygen sensitive liquid products and lyophilized or powdered products. Any modification in the headspace pressure, moisture or oxygen level may result in the degradation of the active drug, as well as in the reduction of drug potency and product shelf life.
Products in contact with air in the container could give rise to bacterial growth or make the content ineffective due to oxidation over time, leading to color change, toxicity and rancidity with negative consequences like ill-health of patients and consumers and poor reputation for the manufacturers.
Pharmaceutical Companies are required to protect their products not only with proper and safe enclosures, but they must also fill the space that surrounds the product (i.e. headspace), with particular gases (e.g. Nitrogen, Carbon Dioxide) or simply maintain a high level of vacuum inside the enclosure, to avoid contact with oxygen or moisture.
Specific requirements for sterile drugs packaged under full or partial vacuum are covered by EU GMP Annexure 1 Manufacture of Sterile Medicinal Products, section 123: “Containers sealed under vacuum should be tested for maintenance of that vacuum after an appropriate, pre-determined period”. The testing method conforms to provisions expressed in United State Pharmacopeia, USP General Chapter “Package Integrity Evaluation – Sterile Products” (USP 39-NF34): Laser-Based Gas Analysis is listed among the Deterministic Leak Test Technologies. Validations and qualifications are easy to perform by means of advanced protocols and documentation.
Headspace Analysis works on the principle of Tunable diode laser absorption spectroscopy (TDLAS), a non-destructive method able to measure the concentration of a certain gas in closed container even at very low detection limits, with a sufficient headspace size to pass through with a laser beam.
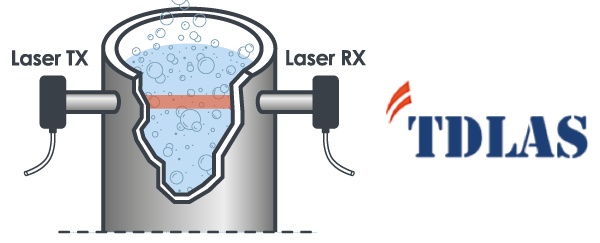
The wavelength diode laser is tuned over the absorption line of the gas under test (760 nm for Oxygen, 1854 nm for Moisture, 2000 nm for Carbon Dioxide) and the intensity of the transmitted radiation is measured. More the gas molecules present in the headspace, less the energy that arrives at the photodiode used to convert light into electrical signals. The transmitted intensity can be related to the gas concentration by the Beer-Lambert law.
It is also possible to apply techniques of signal modulation to further improve the signal-to-noise ratio, ensuring the detection of small variations of signals in front of a wider operating range: WMS (Wavelength Modulation Spectroscopy) and FMS (Frequency Modulation Spectroscopy) are the most common types of modulation techniques.
Learn more about how Headspace Gas Analysis (HGA) works in pharmaceutical products.