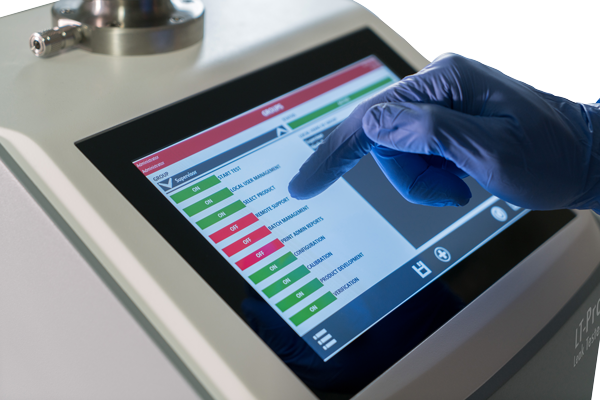
LT-Pro Leak Tester Flexible CCIT benchtop leak tester
Pharmaceuticals
Non-invasive, non-destructive leak tester for laboratory and in-process control applications. Provides accurate and reliable closed container integrity testing (CCIT) for rigid and flexible packaging.
PLAY VIDEO
BFS
Bottles
Carpoules
Cartridges
FFS
Pouches
Prefilled Syringes
Vials
Flowpacks
Cups
Highlights
- Enhanced flexibility
- Interchangeable tooling for testing a wide variety of containers
- Multiple test methods: vacuum and pressure decay
- Available with either 5μm or 1μm test accuracy
- Non-invasive, non-destructive testing of personalized medicine
- Small-batch testing
- Ergonomic design: lightweight, compact, easy to operate, clean, and move.
- Simple Ewon connection makes it easy to store, analyze, and document test results.