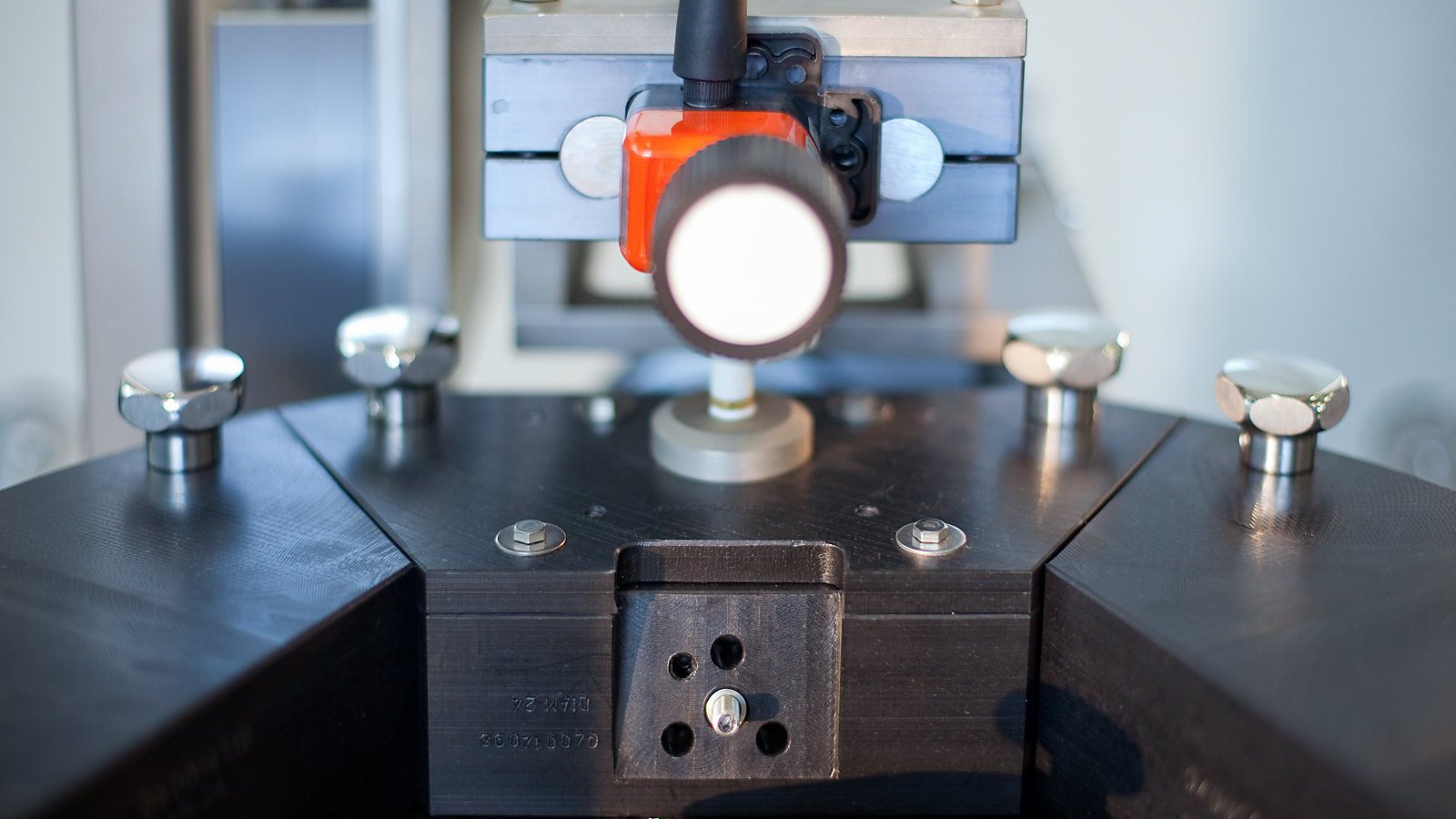
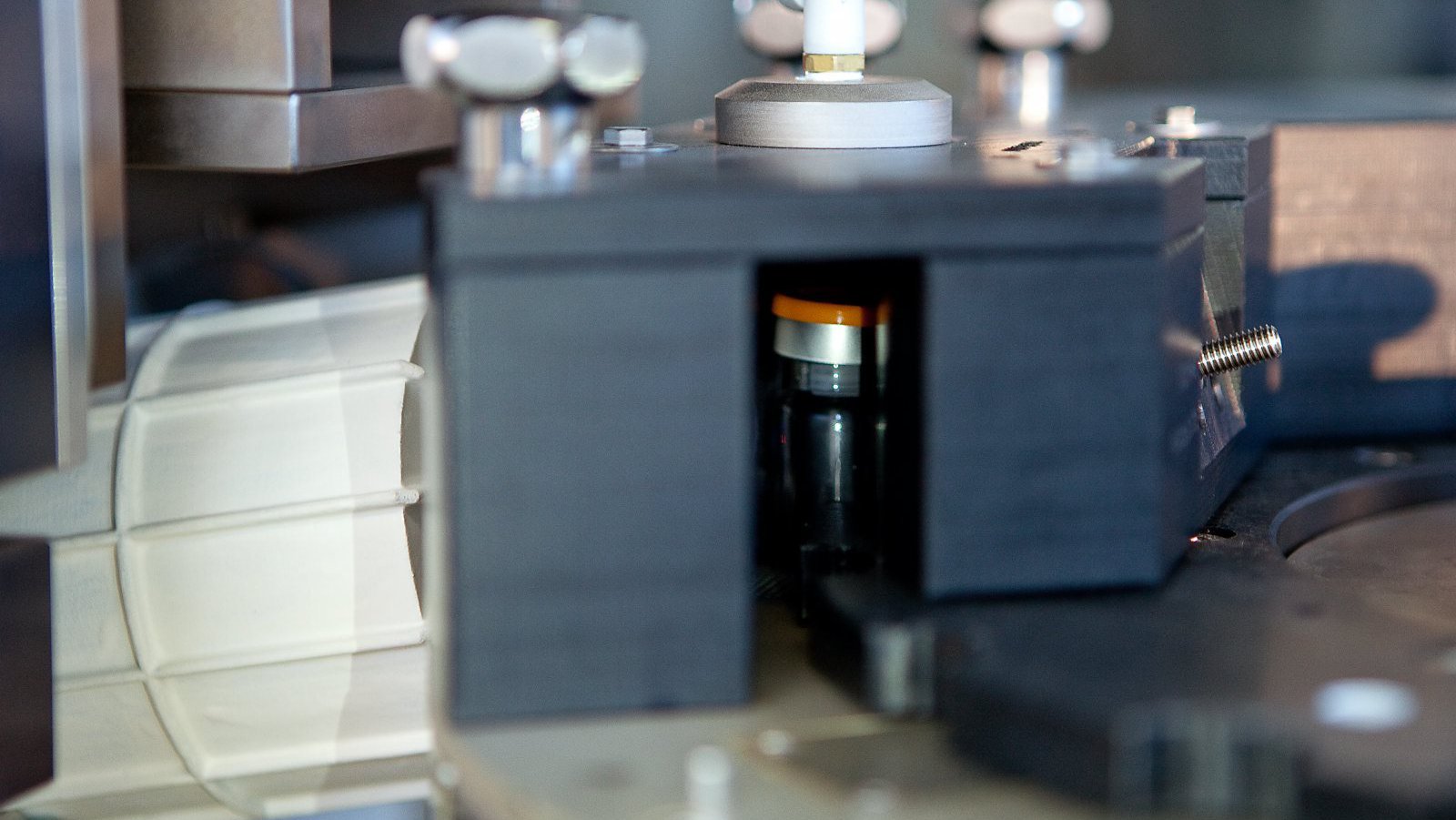
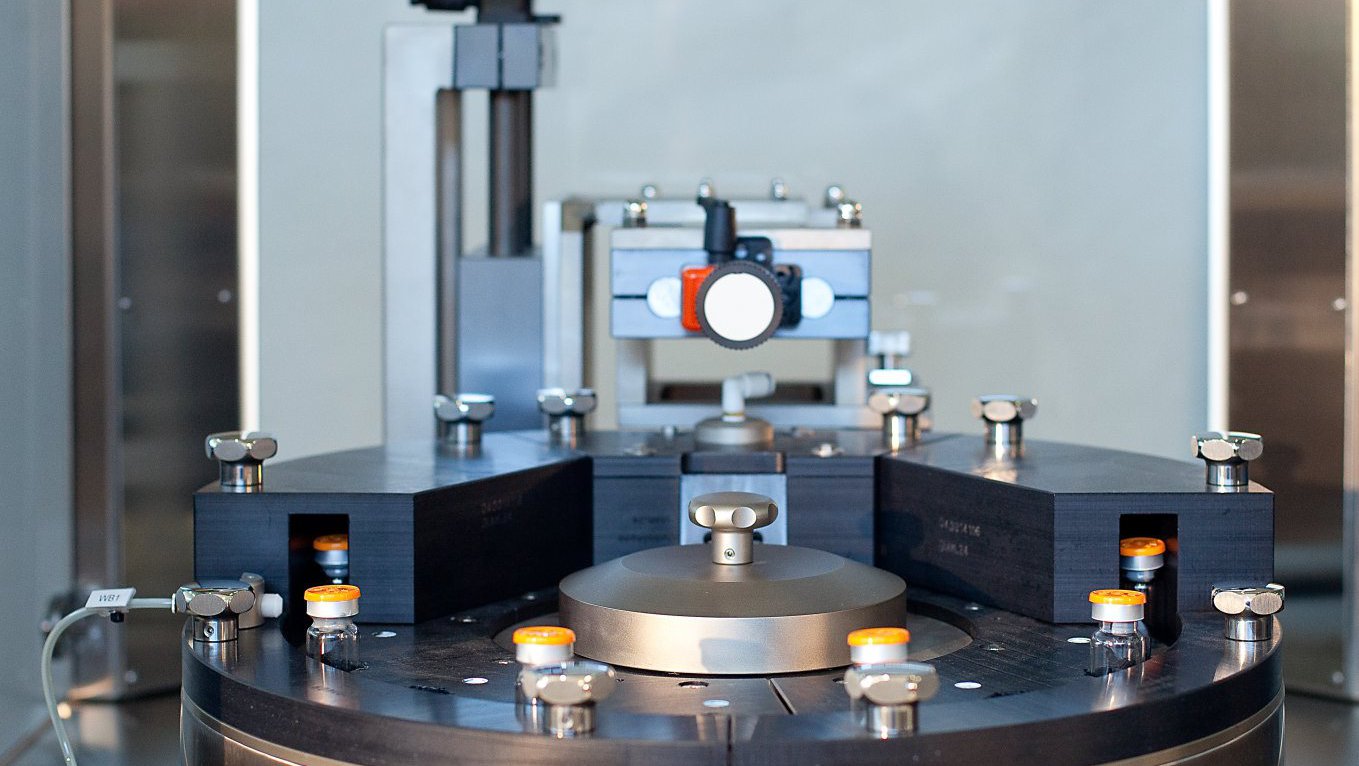
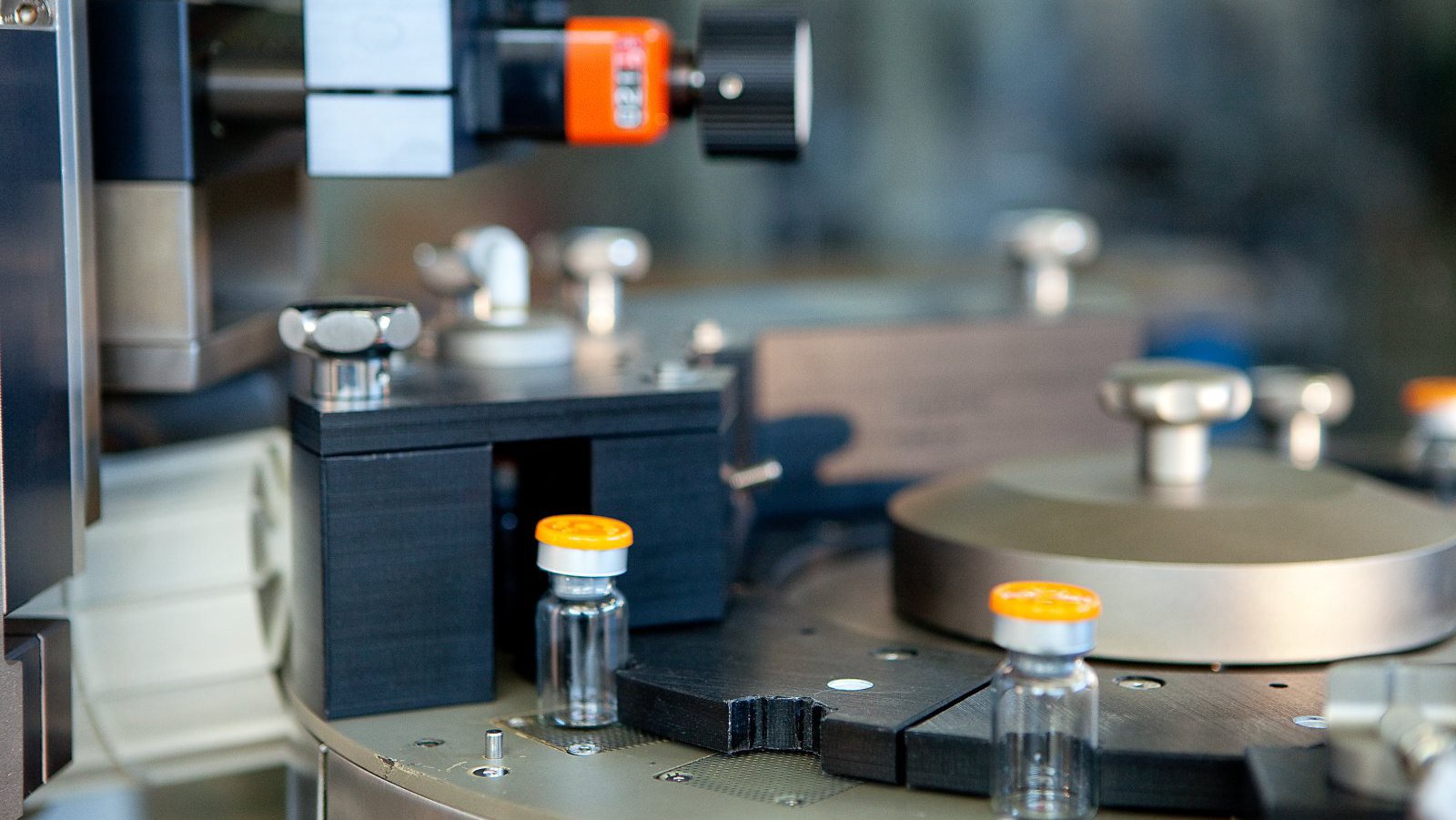
LVA 600 In-Line Headspace Gas Analyzer
Pharmaceuticals
In-line fully automated Integrity Inspection System for performing Headspace Gas Analysis (HGA) of sterile pharmaceutical containers.
PLAY VIDEO
Ampoules
Cartridges
Prefilled Syringes
Vials
Highlights
- Nitrogen purging not required
- No reference container required during operation
- Easily upgradeable
- High Versatility to achieve customizable solutions
- Automatic container rotation ensures highly accurate test results
- Extreme stability and accuracy even with limited headspace
- CFR part 11 compliance and 4.0 full integration