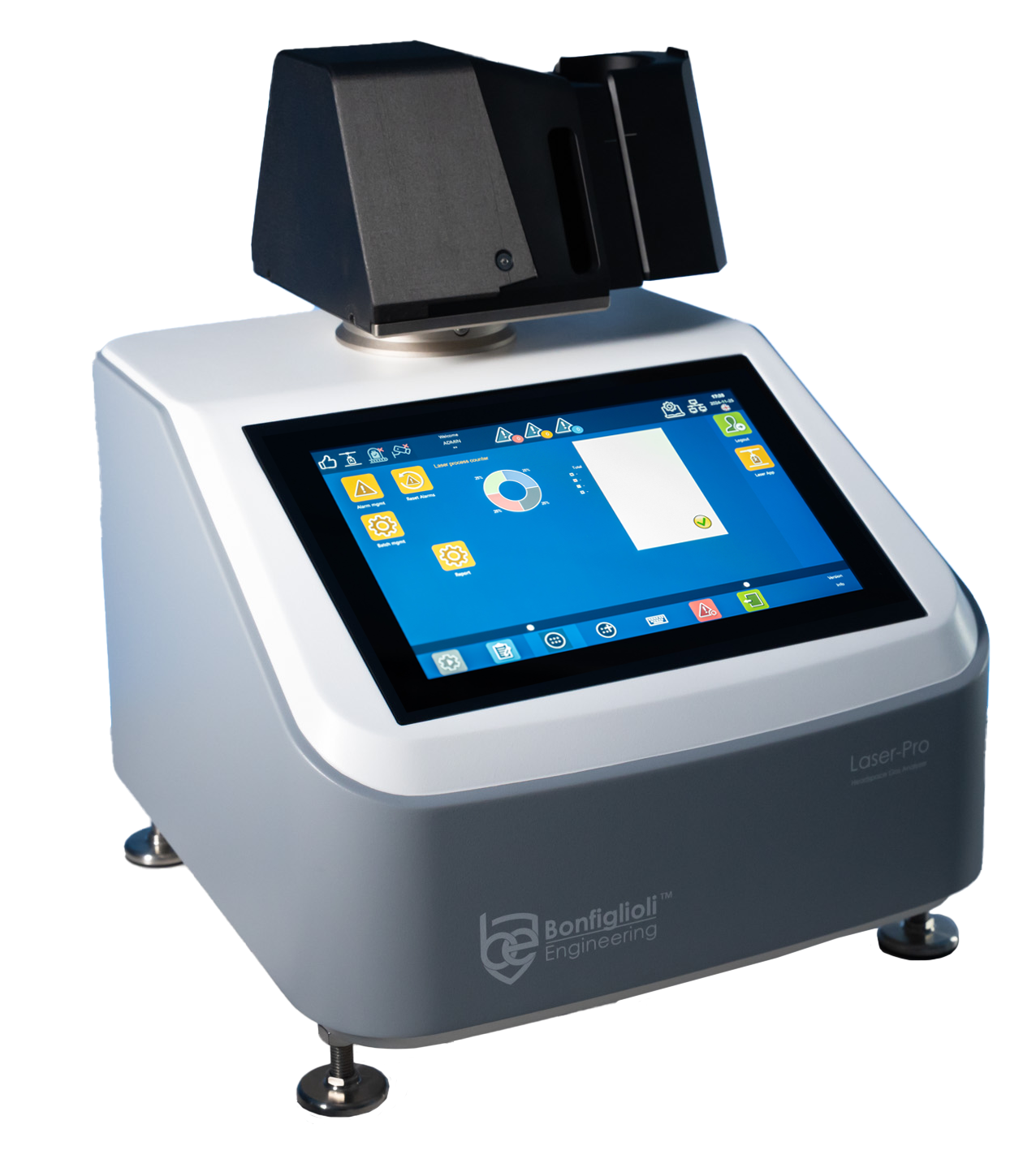
Laser-Pro Next-Gen Headspace Gas Analyzer
Pharmaceuticals
Non-Destructive laboratory headspace gas analyzer measuring specific gas concentration in the headspace of sterile transparent pharmaceutical packaging such as ampoules, vials and plastic containers. Suitable for the following types: liquids, lyo, powder, pastes, solids, or semi-solid content.
PLAY VIDEO
Ampoules
Vials
Highlights
- Flexible, accurate, and easy-to-use solution
- Fast, reliable and repeatable results
- Stable calibration for long-lasting reliability
- Sample-housing unit that minimizes outside influences
- Simple and safe tooling format placement (based on magnetic couplings)
- Nitrogen purging not required