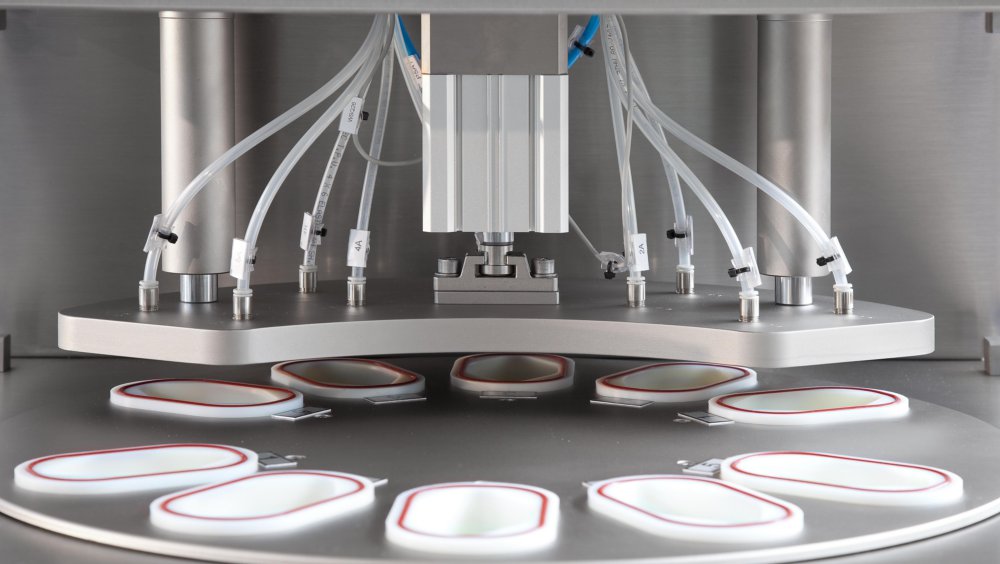
LF-SMH Lab scale multi-head CFR21 Part 11 Compliant
Pharmaceuticals
Non-Invasive, Non-destructive Integrity Inspection System for finished and filled containers for in process and off-line control applications.
PLAY VIDEO
Ampoules
Bottles
Carpoules
Flowrapped Device
IV Bags
MDI
MDPI
Pouches
Prefilled Syringes
Vials
Flowpacks
Highlights
- Quick and sensitive test
- Zero alteration of container features
- Vacuum and positive pressure testing
- Fast, reliable and repeatable results
- Highly functional, intuitive HMI
- Cost-effective solution
- System autodiagnostics available